
We have established methods for the discovery of drugs for treatment of American Trypanosomiasis (Chagas disease), using farnesyl pyrophosphate synthase from Trypanosoma cruzi as a target. To enable the discovery of drugs, it is essential to have access to relevant forms of the target protein and valid biochemical methods for studying the protein and effects of compounds that may be evolved into drugs. This pilot screen confirms that the procedures developed herein enables SPR‐biosensor driven fragment‐based discovery of leads targeting tcFPPS, despite the lack of a reference compound. Instead, ranking could be performed from the slope of the linear relationship at low concentrations. Characterization of hits by SPR showed that all had low affinities and the relationships between steady‐state responses and concentrations were not sufficiently hyperbolic for determination of KD‐values. M > 1☌ and selectivity for tcFPPS in the presence of Mg2+.
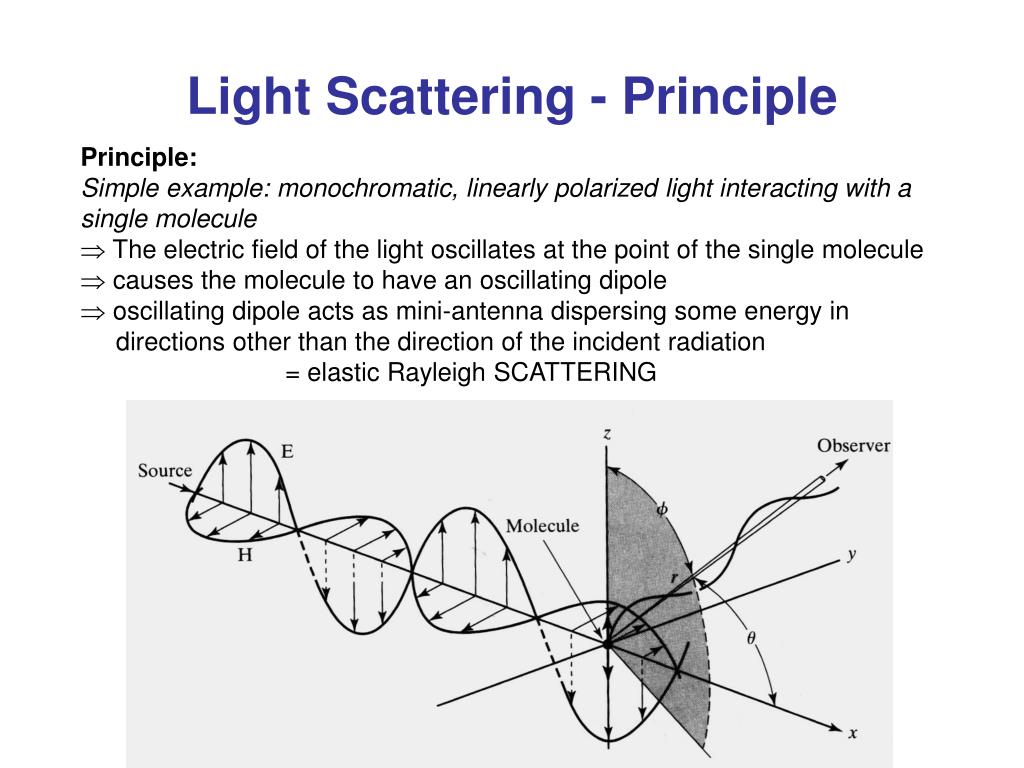
Hits were selected on the basis of response levels or ΔT A library of 90 fragments was efficiently screened by both assays at a single concentration in the presence and absence of the catalytic cofactor Mg2+. A thermal shift assay was used in parallel. An SPR‐biosensor assay for fragment library screening and hit confirmation was developed. It also confirmed that sensor surfaces prepared with structurally intact protein was active. A new coupled enzymatic assay, using luciferase for detection of pyrophosphate, was developed and used to confirm that the purified enzyme was active after purification and storage. The improved methods enabled the production of pure, folded and dimeric protein, and identified procedures for storage and handling. Initial problems with protein stability and lack of useful reference compounds motivated optimization of experimental procedures and conditions. Application of Dynamic Light Scattering (DLS) to Protein Therapeutic Formulations: Principles, Measurements and Analysis - 1.
.jpg)
The method requires functional sensor surfaces with high sensitivity for extended times and appropriate controls. Per sample, analytical column, 0.Procedures for producing and exploring Trypanosoma cruzi farnesyl pyrophosphate synthase (tcFPPS) for surface plasmon resonance (SPR) biosensor‐driven fragment‐based discovery have been established. Per buffer, includes analytical column equilibration, BSA control, in addtion to per-sample fee. Per sample fee, in duplicate wells, 5-10 acquisitions/scan, 2 scans/well, in addition to plate setup fee. In addition to per-sample fee.ĭLS - sample data collection - macromolecules Setup fee per plate, up to 48 samples, includes BSA control and 1 buffer control. Required for samples with high molecular weight modifiers, such as glycosylation or detergent micelle will require additional analysisĭn/dc measurements of detergent or protein samples Hydrodynamic Radius from DLS (when light scattering signal is sufficient) Dynamic light scattering characterizes particle size by shining an incident light source, typically a monochromatic laser beam, into a sample. Particle concentration, type of ions in the medium can. Includes:Ĭolumn equilibration with your buffer (pH at or below pH 7.5) or with PBSīSA Standard to test column performance and normalize detectors DLS can be used to characterize the size of various particles including proteins and small compounds.
Prior purification by size-exclusion chromatography (SEC) is strongly recommended. Size-exclusion Chromatography with Multi-Angle Light Scattering (SEC-MALS)ĬMI SEC-MALS System: Wyatt Dawn Heleos with in-line DLS, RI UV, Agilent chromatography Standard SEC-MALS Serviceįor proteins and protein complexes between 15 KDa and 1 MDa, without high molecular weight modifiers (modifiers of mass >5% total).


 0 kommentar(er)
0 kommentar(er)
